Understanding What is an Atom: The Building Blocks of Matter
In the quest to understand the universe and everything within it, the concept of the atom is fundamental. But what exactly is an atom, and why is it so crucial to science? This article delves into the heart of atomic theory, exploring the intricate details of what constitutes an atom, its historical significance, and its role in the modern scientific landscape.
Key Takeaways
- An atom is the smallest unit of matter that retains the properties of an element.
- Atoms consist of a nucleus made up of protons and neutrons, with electrons orbiting this nucleus.
- Understanding atoms is crucial for fields such as chemistry, physics, and various branches of engineering.
- The study of atoms has led to significant technological and scientific advancements.
What is an Atom?
At its core, an atom is the smallest constituent unit of ordinary matter that has the properties of a chemical element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are incredibly small, typically around 100 picometers in size, which is a billionth of a meter. Despite their minuscule size, atoms are the building blocks of everything we see around us.
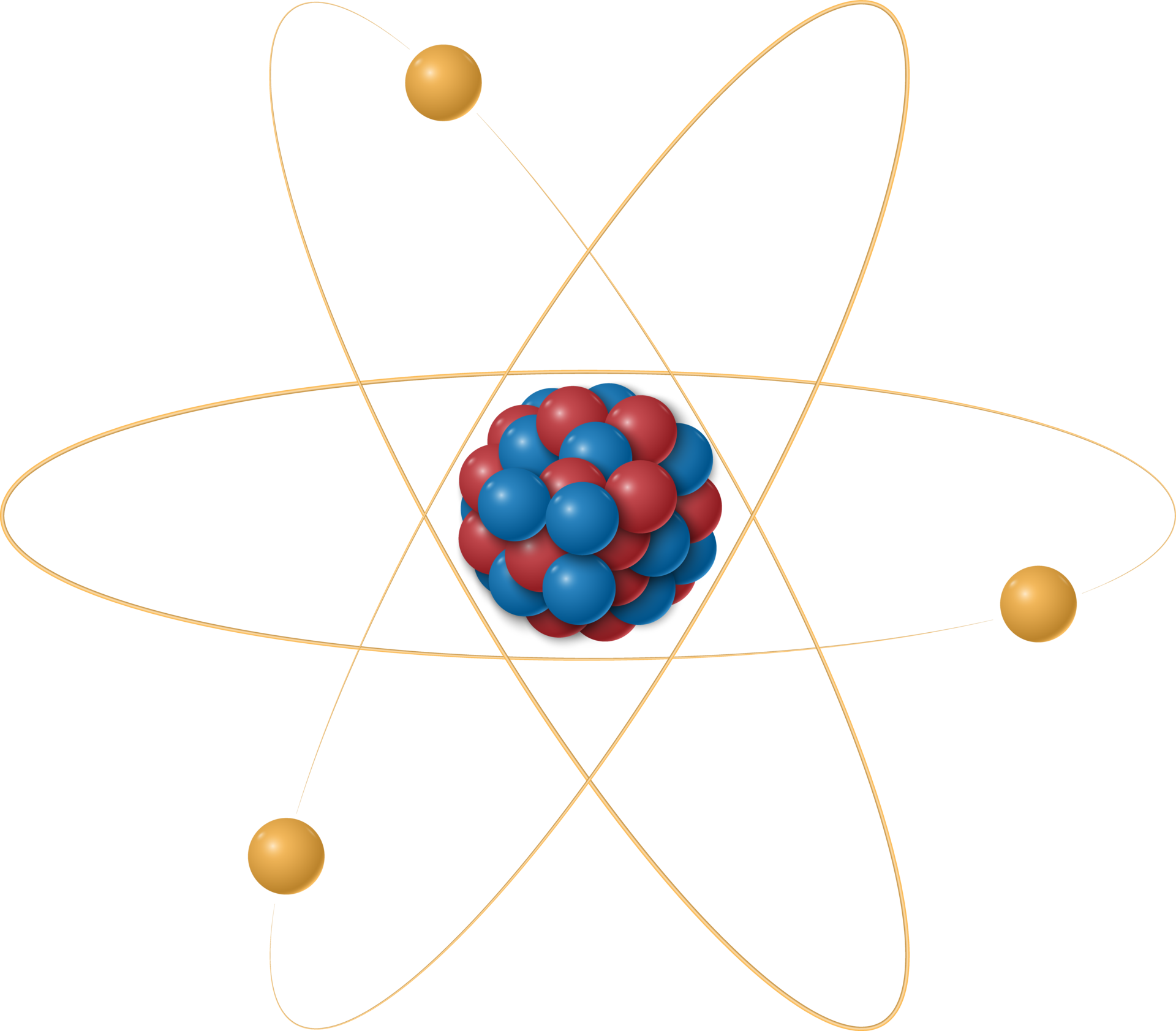
The Structure of an Atom
Understanding the structure of an atom is crucial to grasping its function and importance. An atom consists of a central nucleus surrounded by one or more electrons. The nucleus itself contains protons and neutrons.
- Protons: These are positively charged particles found within the nucleus. The number of protons in an atom’s nucleus defines the chemical element of the atom, known as the atomic number.
- Neutrons: Neutrons are neutral particles that also reside in the nucleus. They contribute to the atomic mass but do not affect the chemical properties of an element.
- Electrons: Electrons are negatively charged particles that orbit the nucleus in various energy levels or shells. The arrangement of electrons determines the atom’s chemical properties and reactivity.
The History of Atomic Theory

The concept of the atom dates back to ancient Greece, where philosophers like Democritus theorized that matter was composed of small, indivisible particles. However, it wasn’t until the 19th century that scientific evidence began to support these ideas. John Dalton formalized atomic theory in the early 1800s, proposing that each element consisted of identical atoms and that chemical reactions involved the rearrangement of these atoms.
Later, discoveries by scientists such as J.J. Thomson, Ernest Rutherford, and Niels Bohr further refined our understanding of atomic structure. Thomson’s discovery of the electron, Rutherford’s nuclear model of the atom, and Bohr’s model of electron orbits were pivotal in shaping modern atomic theory.
Atoms in Modern Science
Today, our understanding of atoms is more sophisticated than ever. Quantum mechanics provides a framework for understanding the behavior of electrons within atoms, leading to the development of technologies such as semiconductors, lasers, and nuclear power. The study of atoms and their interactions is fundamental to fields like chemistry, physics, materials science, and engineering.
In chemistry, the concept of the atom is essential for understanding chemical reactions, bonding, and molecular structure. In physics, atomic theory underpins the study of matter and energy, while in engineering, knowledge of atomic interactions is crucial for developing new materials and technologies.
The Importance of Studying Atoms
Studying atoms is not just an academic pursuit; it has practical implications that touch every aspect of our lives. From the development of new materials and medicines to advancements in energy production and electronics, the knowledge gained from atomic research has a profound impact on society.
For instance, the development of nuclear energy relies on our understanding of atomic nuclei and the forces that hold them together. Similarly, the electronics industry depends on our ability to manipulate electrons within atoms to create semiconductors and integrated circuits.
the atom is a fundamental component of the universe, serving as the building block for all matter. Understanding what an atom is and how it functions is crucial for advancing science and technology. From ancient philosophical musings to cutting-edge research, the study of atoms continues to be a central theme in our quest to understand the natural world. As we continue to explore the mysteries of the atom, we unlock new possibilities for innovation and discovery, shaping the future of technology and science.